This website is best viewed using the horizontal display on your tablet device.
POETYK PSO-1 Study Design
52-week, double-blind, placebo- and active comparator-controlled study in moderate-to-severe plaque psoriasis1-3
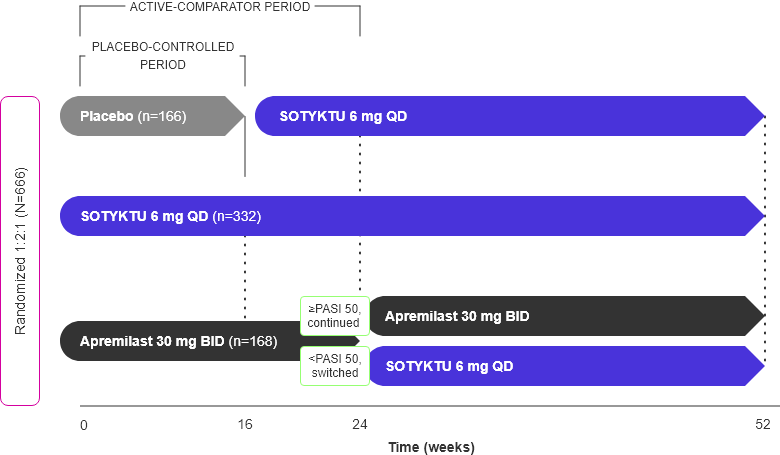
Adapted from Product Monograph and Armstrong et al., 2022.
- Co-primary endpoints1
- % patients who achieved PASI 75 vs. placebo at Week 16
- % patients who achieved a sPGA score of 0 (clear) or 1 (almost clear) vs. placebo at Week 16
- Secondary endpoints1
- % patients who achieved PASI 90, PASI 100, sPGA 0, ss-PGA (scalp) score of 0 (clear) or 1 (almost clear) vs.
placebo at Week 16 - % patients who achieved PASI 75, PASI 90, and sPGA 0/1 vs. apremilast at Weeks 16 and 24
- % patients who achieved sPGA 0 and ss-PGA 0/1 vs. apremilast at Week 16
- Key inclusion criteria1,2
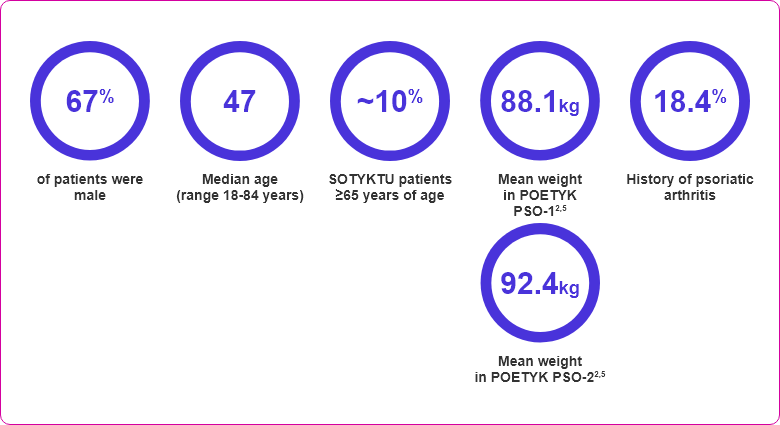
- ≥18 years of age
- Moderate-to-severe plaque psoriasis
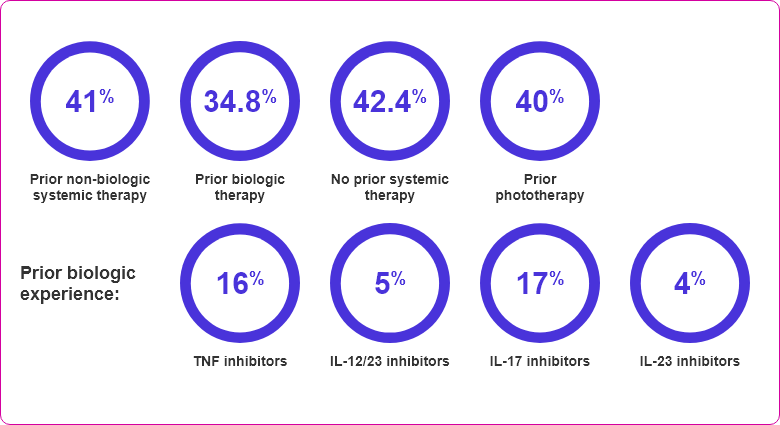
- Eligible for systemic therapy or phototherapy
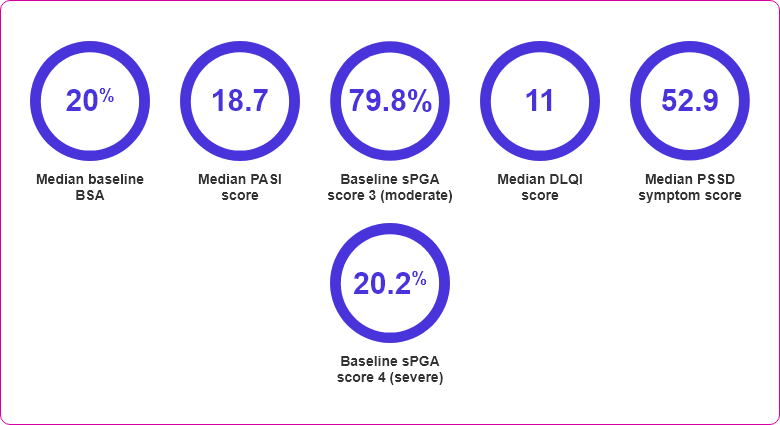
- BSA involvement ≥10%
- PASI score ≥12
- sPGA ≥3 (moderate or severe) on a 5-point scale of overall disease severity
- Key exclusion criteria3
- Has non-plaque psoriasis (i.e., guttate, inverse, pustular, erythrodermic, or drug-induced psoriasis)
at screening or Day 1 - History or evidence of outpatient active infection and/or febrile illness within 7 days before Day 1
- Prior exposure to SOTYKTU or active comparator
POETYK PSO-2 Study Design
52-week, double-blind, placebo- and active comparator-controlled study
in moderate-to-severe plaque psoriasis1,4
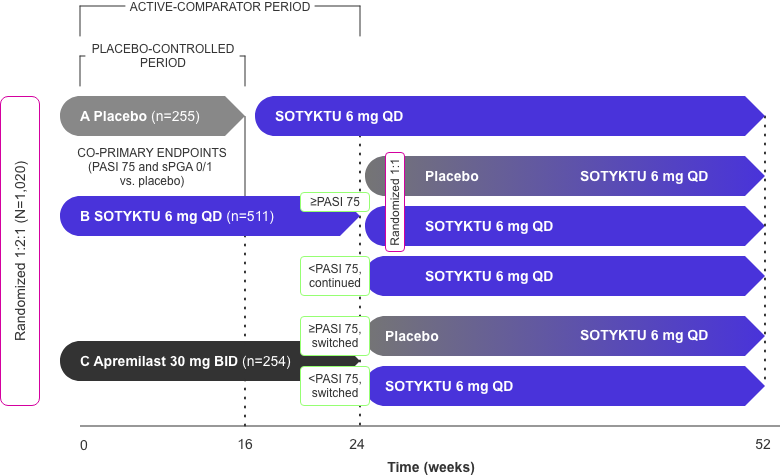
Adapted from Product Monograph and Strober et al., 2022.
- Co-primary endpoints1
- % patients who achieved PASI 75 vs. placebo at Week 16
- % patients who achieved a sPGA score of 0 (clear) or 1 (almost clear) vs. placebo at Week 16
- Secondary endpoints1
- % patients who achieved PASI 90, PASI 100, sPGA 0, ss-PGA (scalp) score of 0 (clear) or 1 (almost clear) vs.
placebo at Week 16 - % patients who achieved PASI 75, PASI 90, and sPGA 0/1 vs. apremilast at Weeks 16 and 24
- % patients who achieved sPGA 0 and ss-PGA 0/1 vs. apremilast at Week 16
- Key inclusion criteria1,4
- ≥18 years of age
- Moderate-to-severe plaque psoriasis
- Eligible for systemic therapy or phototherapy
- BSA involvement ≥10%
- PASI score ≥12
- sPGA ≥3 (moderate or severe) on a 5-point scale of overall disease severity
- Key exclusion criteria4,5
- Has non-plaque psoriasis (i.e., guttate, inverse, pustular, erythrodermic, or drug-induced psoriasis)
at screening or Day 1 - History or evidence of outpatient active infection and/or febrile illness within 7 days before Day 1
- Prior exposure to SOTYKTU or active comparator