This website is best viewed using the horizontal display on your tablet device.
PASI
SOTYKTU demonstrated significantly higher PASI 90 response rates vs. apremilast at Weeks 16 and 24 (secondary endpoints)1
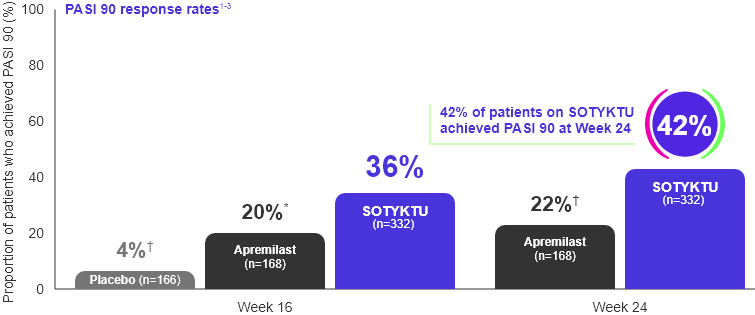
Adapted from Product Monograph and Armstrong et al., 2022.
* p<0.001 SOTYKTU vs. apremilast
† p≤0.0001 SOTYKTU vs. placebo or SOTYKTU vs. apremilast
SOTYKTU demonstrated significantly higher PASI 75 response rates vs. apremilast at Weeks 16 and 24 (secondary endpoints)1
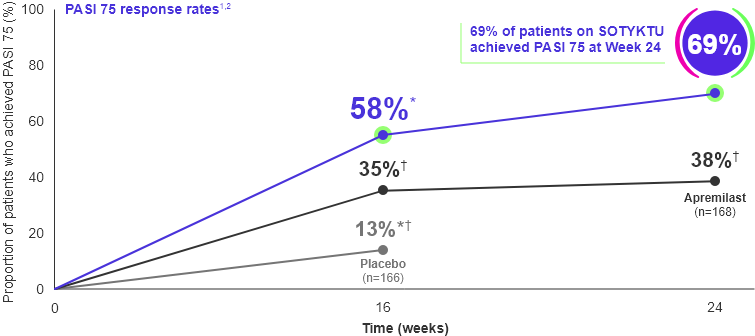
Adapted from Product Monograph and Armstrong et al., 2022.
* Co-primary endpoint SOTYKTU vs. placebo
† p≤0.0001 SOTYKTU vs. placebo or SOTYKTU vs. apremilast
Examination of age, sex, ethnic origin, body weight, duration of disease, baseline disease severity, and previous treatment with biologic or non-biologic agents did not identify differences in response to SOTYKTU among these subgroups.1
Durability data
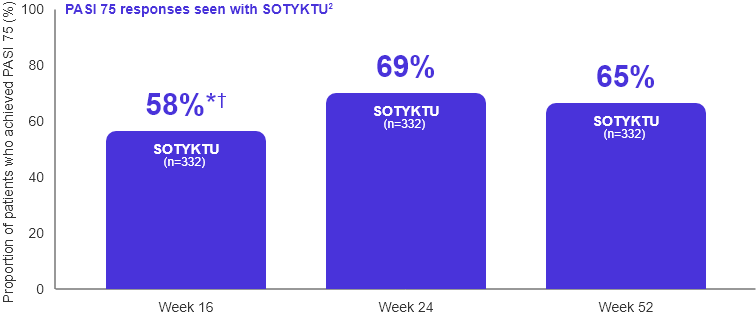
Adapted from Armstrong et al., 2022.
* Co-primary endpoint SOTYKTU vs. placebo
† p≤0.0001 SOTYKTU vs. placebo
- 65% of patients on SOTYKTU achieved PASI 75 at Week 52
- Among patients who received SOTYKTU and achieved PASI 75 at Week 24, 81% of patients who continued on SOTYKTU maintained PASI 75 response at Week 521
- Among patients who received SOTYKTU and achieved PASI 90 at Week 24, 74% of patients who continued on SOTYKTU maintained PASI 90 response at Week 521
Scalp
Most (70.3%) SOTYKTU patients achieved an ss-PGA (scalp) score of 0 (clear)
or 1 (almost clear) at Week 16 (secondary endpoints)1,2
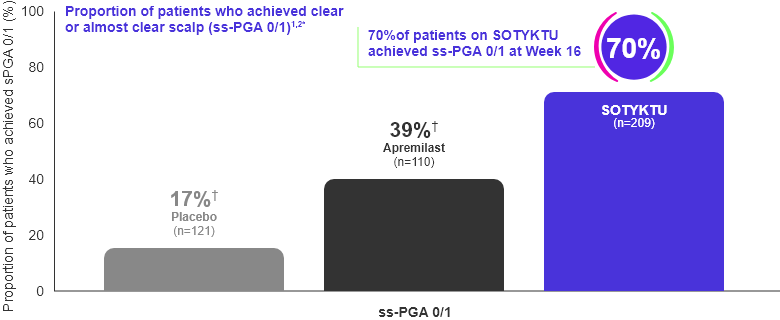
Adapted from Product Monograph and Armstrong et al., 2022.
* Includes patients with baseline ss-PGA scores ≥3.
† p≤0.0001 SOTYKTU vs. placebo or SOTYKTU vs. apremilast
DLQI
Significantly more SOTYKTU patients achieved a DLQI score of 0/1 at Week 16 vs. placebo (secondary endpoint)1,2
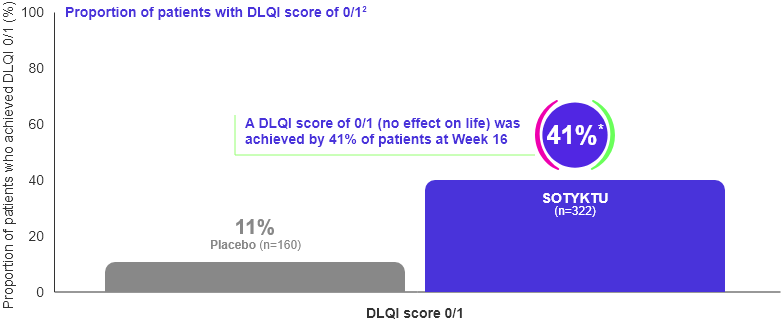
Adapted from Armstrong et al., 2022.
* p<0.0001