This website is best viewed using the horizontal display on your tablet device.
POETYK PSO-1 and PSO-2
Adverse reactions occurring in ≥1% of the SOTYKTU group with
higher rates than placebo, through Week 16 (pooled data)1
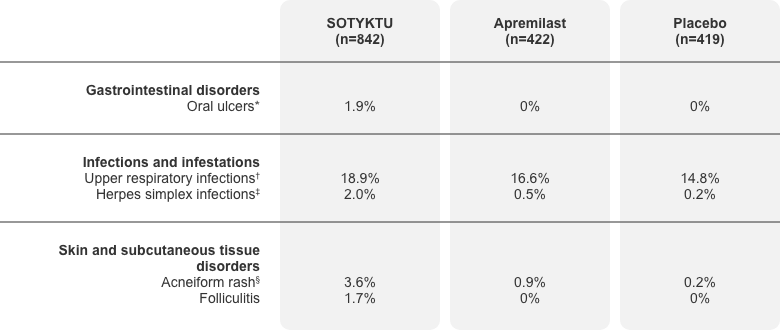
Adapted from Product Monograph.
- Less Common Adverse Reactions
- Herpes zoster was reported in SOTYKTU-treated patients at an incidence of <1% and >0.1%
- Malignancies have been reported in SOTYKTU-treated patients. The potential role of SOTYKTU in the development of malignancies is unclear.
- No new adverse reactions were identified with SOTYKTU through Week 52 and the incidence of common adverse reactions did not increase compared to those observed during the first 16 weeks of treatment
- Discontinuation rate due to adverse events was 2.4% for SOTYKTU vs. 3.8% for placebo and 5.2% for apremilast. The majority of events leading to treatment discontinuation occurred in single patients